On December 1st, Adoela Onasanya will defend her INSPiRED PhD Thesis titled- Designing for Neglected Tropical Diseases: Co-creating digital diagnostic devices for Low-Resource Settings
You can download her thesis here. The summary of her PhD Thesis:
The need for new diagnostics for NTDs
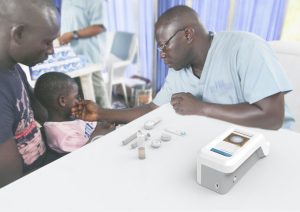
Neglected Tropical Diseases (NTDs) are a group of infections affecting more than 2 billion and are a cause of significant morbidity and mortality. Despite their significant burden, NTDs are frequently disregarded in the research and development of new diagnostics, medications, and vaccines. New digital diagnostics for neglected tropical diseases (NTDs) are needed, as the current mainstay of diagnosis for most parasitic NTDs, the microscope, is often unavailable or requires expertise not readily available at the primary level of care. Digital optical diagnostics can fill this role for affordable and user-friendly NTD diagnostic devices that can facilitate NTD treatment and monitoring and evaluation thereby contributing to improved healthcare outcomes. Designing these products necessitates an understanding of the social and healthcare context in which they will be used, the design process and the diagnostic device design, which require collaborating with stakeholders in the relevant use setting. The main objective of this thesis is to investigate how smart optical diagnostics for NTDs can be created for endemic countries by integrating local experience and knowledge leading to local system uptake.
The use context of digital diagnostics for NTDs
From this thesis, it was shown that the use context is the environment in which a diagnostic device will be used and it is a complex system that involves interactions between the physical, social, cultural, and environmental setting, including healthcare organizational goals, policies, workflow tasks, resources, and users. The use context includes the social and healthcare context which is interconnected and involves individuals, communities, and institutions. The social context influences awareness, and interest in prevention, diagnosis, and treatment, as well as the availability, accessibility, affordability, accommodation, and acceptability of resources (human, infrastructural, and material) at the individual, community, and institutional levels. Collaboration and coordination among stakeholders in the use context is important for the outcome of the design process, the diagnostic device design, and the adoption of the diagnostic device. This is important for resource allocation, alignment with national and international strategies, and the development of new diagnostics.
The design process for developing an NTD diagnostic device
The design process for developing an NTD diagnostic device in this thesis is a 5-step iterative process that includes empathizing, defining, ideating, prototyping, and testing. It was shown that co-creation with stakeholders is crucial for the design process because it fosters collaboration between groups that may not typically be involved in product design, leading to a shared understanding of the disease process and raising awareness of challenges and opportunities in the diagnostic landscape. Several tools for stakeholder mapping and analysis for co-creation include social network analysis, q-methodology, document reviews and interviews.
The diagnostic device design process for NTD diagnostics
The design of a diagnostic device is an iterative process that requires input from stakeholders in five key areas including the overall design and evaluation of the device, the software system, the hardware system, the structural composition, specification, and style of the device, and the product manual. The diagnostic device design process also required co-creation with a wide range of stakeholders crucial for developing product specifications.
The outcome of the thesis shows two main categories of product specifications: broad-based and context-based exist. Broad-based specifications include technical, regulatory, and business specifications. Technical specifications can be divided into two categories: manufacturing and performance-based. Manufacturing specifications relate to the materials, electrical and machinery needed to give the product form and function, while performance-based specifications are related to functionality with specific requirements such as sensitivity, specificity, and speed. Regulatory specifications relate to standards set by regulatory bodies such as the International Standards Organization (ISO) and the World Health Organization (WHO). Business-based specifications relate to financing and comparing new diagnostics to current models of diagnosis. Manufacturing specifications have a strong influence on the business case for new diagnostics, especially in countries where funding for NTDs is not a priority.
Context-based specifications are associated with contextual factors that may affect user experience, usability, and acceptability within the context of use. Context-based specifications can influence manufacturing and business specifications.
Based on the interrelatedness of the two categories of product specifications, different product use scenarios for NTD diagnostics were created. In the Nigerian context, three potential use scenarios for new NTD diagnostics include screening programs, monitoring and evaluation, and point-of-care testing.
Adopting new diagnostics for NTDs
The adoption of new diagnostic devices for Neglected Tropical Diseases (NTDs) is a complex process that involves various stakeholders and stages such as research and evaluation, training and education, implementation and integration, monitoring and evaluation, support and maintenance, device evaluation and improvement, and scale-up. Understanding the context and contextual factors at all stages is crucial in designing an adoption process for digital diagnostics for NTDs in low-resource settings. Scarce resources, lack of infrastructural support, political instability, and cultural differences can affect adoption and scale-up. Therefore, conducting a thorough needs analysis and involving culturally-aware stakeholders can help identify unique difficulties within the developing context and undertake a cost and feasibility analysis.
The diagnostic device and its implementation process have a dynamic relationship with the context, and it is important to assess the perceived need for the innovation to be implemented, its potential compatibility with existing routines, and an assessment of user expectations during the design process and diagnostic device design. To positively affect the adoption of NTD diagnostics in low-resource settings, a multifaceted strategy involving cooperation between stakeholders at various levels can be employed. Engaging local stakeholders and communities, encouraging collaboration between national and sub-national governments, non-governmental organizations, research institutions, and the private sector, providing adequate training and support to healthcare workers, addressing regulatory and policy barriers, undertaking multiple proofs of concept studies, and monitoring and evaluating diagnostics used during implementation are some possible strategies to ensure device adoption.
Within the Sub-Saharan African context, helminthic NTD elimination requires a systemic design approach involving zooming in and out of the context of use, design process, and diagnostic device design while managing and co-creating with stakeholders to ensure the development and adoption of newly developed digital diagnostic devices for NTDs. By following these strategies, the development and adoption of NTD diagnostic tests that are contextually fit, useful, and acceptable can lead to an uptake in the local context.